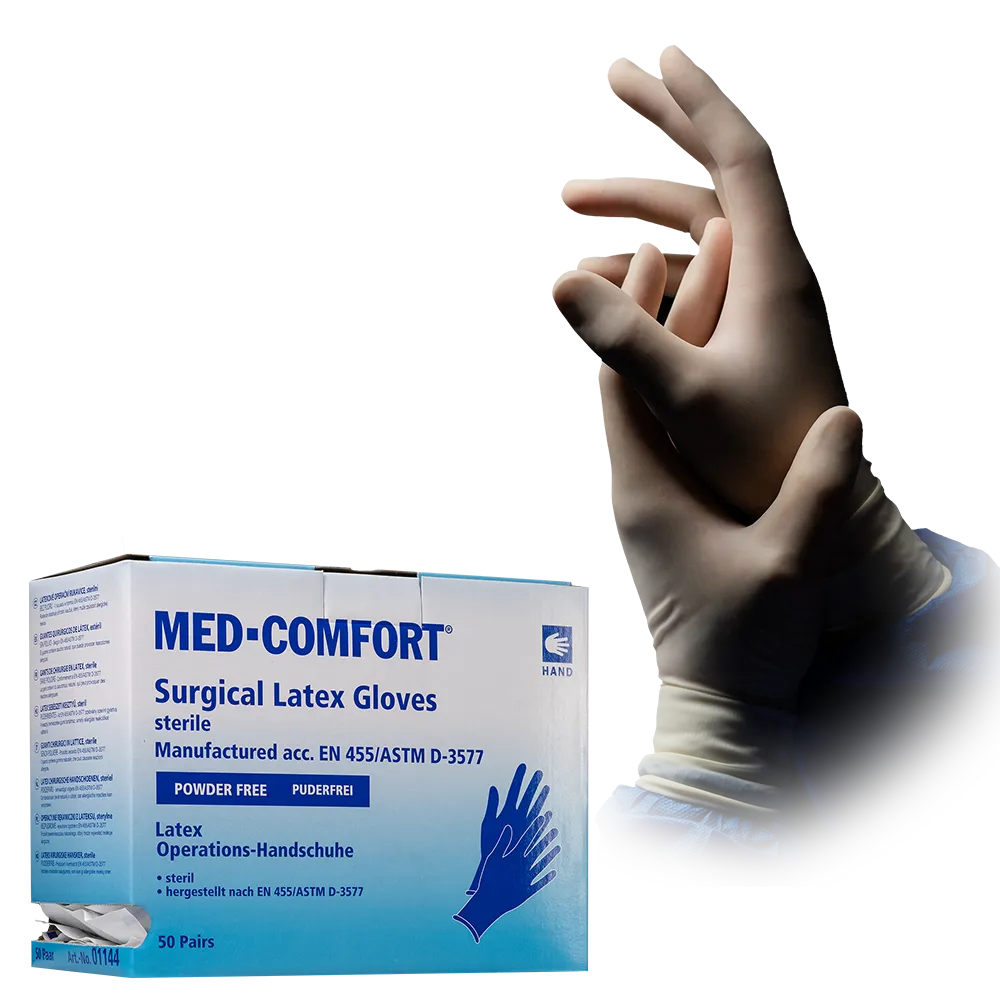
Sterile Surgery Gloves
- 5,5 (on request)
- 7
- 8
- 6
- 7,5
- 6,5
- 8,5
- 8,5
- 6,5
- 8
- 7
- 7,5
Sterile surgical gloves – Single‑use surgical gloves
- 1. What are sterile surgical gloves?
- 2. Materials & properties of sterile surgical gloves
- 3. Sterilisation, packaging & application technique
- 4. Standards & certifications
- 5. Benefits of sterile single‑use surgical gloves
- 6. Typical application areas of sterile surgical gloves
- 7. FAQ on sterile surgical gloves
1. What are sterile surgical gloves?
Sterile surgical gloves – also referred to as surgical gloves – are specifically designed medical single‑use gloves used in the operating theatre and other sterile working environments. They serve as a microbiological barrier, protecting both patients and medical personnel, and are an essential component of any sterile surgical setup. Their sterility is ensured through a validated process – typically via ETO sterilisation – and maintained until use by means of individual pair packaging.
In contrast to non‑sterile examination gloves, which are mainly used for routine procedures, diagnostic tasks or cleaning, sterile surgical gloves meet significantly stricter requirements in terms of fit, material quality, tear resistance, barrier protection and regulatory compliance. They are classified as medical devices in Class IIa, and are therefore subject to extended requirements for safety, performance and clinical evaluation.
A key characteristic of surgical gloves is their hand‑specific form (left/right), which follows the natural anatomy of the hand. This enables fine movements, precise instrument handling and fatigue‑free performance during surgery. The cuff length is also longer than that of standard disposable gloves, ensuring the glove reliably overlaps the surgical gown for seamless protection.
Note on powdered and powder‑free variants
Sterile surgical gloves are generally offered in two variants:
Powder‑free is today the international standard, offering superior skin compatibility and suitability for lengthy procedures. This variant avoids the risk of powder‑associated complications such as granulomatous reactions or additional irritation.
Powdered gloves are still used in some specific applications — for instance, when very quick donning is required. However, due to regulatory developments, this variant is increasingly rare.
Both variants undergo the same stringent requirements for sterility and material quality. The differences lie primarily in donning comfort, moisture management and dermatological aspects — which are detailed in section 2.4.
2. Materials & properties of sterile surgical gloves
The material quality of sterile surgical gloves is crucial for safety, ergonomics and precision during surgical procedures. Unlike non‑sterile examination gloves, surgical gloves are manufactured hand‑specific (left/right), feature a finely tuned wall thickness, are anatomically pre‑shaped and possess a extended cuff that reliably overlaps the surgical gown.
The combination of material selection, manufacturing technique and compliance with standards ensures that surgical gloves maintain a stable barrier under stress while permitting sensitive, precise work.
2.1 Latex as preferred material
Latex has been used for many years in surgical settings and remains the benchmark material for surgical gloves. It combines properties that are difficult to replicate fully with synthetic alternatives:
- Excellent elasticity for precise movements
- Superb tactile sensitivity, ideal for delicate instrument work
- High tear resistance even under significant stretching
- Natural adaptability, supporting an exact fit
- Reliable barrier against pathogens and bodily fluids
The excellent combination of control, stability and comfort makes latex the preferred choice for many surgeons.
At the same time, it is important to note that natural rubber latex can trigger Type I latex allergies — users with a diagnosed allergy must switch to synthetic alternatives.
2.2 Alternative materials (synthetic)
Although AMPri mainly offers latex products in the field of surgical gloves, there are several synthetic materials on the market that serve as alternatives. These include:
- Polyisoprene – a synthetic substitute for latex with very good elasticity
- Polychloroprene – high tear resistance, good chemical resistance
- Synthetic rubber blends – for facilities maintaining fully latex‑free operating rooms
These materials also offer high barrier performance and favourable wearing characteristics, though they may not always match the natural tactile sensitivity and elasticity of high‑quality latex gloves.
2.3 Anatomical fit & sizes
The fit of surgical gloves differs significantly from that of general examination gloves:
- Hand‑specific design: Each glove is made as distinct left and right.
- Anatomical pre‑shaping: The natural curvature of the hand is taken into account during manufacturing.
- Finely graded sizes: Typically available in half sizes (e.g. 6.0 / 6.5 / 7.0 / 7.5 / 8.0 / 8.5).
- Optimised grip: Micro‑texturing facilitates secure handling of surgical instruments.
- Extended cuffs: At least 280 mm to ensure secure overlap with the surgical gown.
This combination reduces fatigue and enables precise movements throughout the entire procedure.
2.4 Powder‑free vs. powdered – the two glove types
Sterile surgical gloves are available in two fundamental versions that differ in handling and skin compatibility. Both variants meet the strict requirements for sterility and barrier performance, but they differ in donning technique, regulatory classification and comfort.
| Feature | Powder‑free | Powdered |
|---|---|---|
| Wearing comfort | Very comfortable, even during prolonged use | Comfortable, especially easy to don |
| Donning / doffing | Polymer coating enables good donning performance | Extremely easy donning due to powder |
| Skin compatibility | Very good; no powder‑related skin irritation | Increased risk of irritation and powder‑associated reactions |
| Regulation | Current standard in surgical settings | Internationally restricted in some areas; usage decreasing |
| Suitability for allergies | Preferred for sensitive or irritated skin | May increase risk of allergic or reactive responses |
| Typical applications | Standard for surgical procedures, longer operations | Specialised applications focusing on rapid donning |
In summary: Powder‑free surgical gloves are the established standard in modern surgical workflows, as they are skin‑friendly and best comply with current regulatory requirements. Powdered variants still offer advantages in terms of rapid donning, but are increasingly subject to restrictions and used less often.
3. Sterilisation, packaging & application technique
Safe use of sterile surgical gloves depends on validated sterilisation, reliable packaging, and correct donning and doffing techniques. Each step is a critical part of infection prevention and governed by strict hygienic protocols.
3.1 Sterilisation (ETO) – validated sterility
Sterile surgical gloves are sterilised through a carefully controlled process, frequently using ETO sterilisation (ethylene oxide). This method ensures high microbiological safety while preserving the integrity of the material.
Key advantages of ETO sterilisation:
- Deep penetration into complex material structures
- Suitable for temperature‑ and moisture‑sensitive products
- Internationally recognised and strictly validated
Following sterilisation, aeration cycles ensure reliable elimination of any residual substances.
3.2 Packaging – protection until the operating theatre
The packaging is designed to preserve sterility throughout storage and transport.
Typical features:
- Individual pair packaging for left and right gloves
- Inner sterile peel‑pack packaging
- Robust outer packaging for transport and storage
- Clear labelling of size, batch number, expiry date and sterilisation method
This double packaging allows sterile presentation in the operating theatre and prevents contact with non‑sterile surfaces.
3.3 Donning techniques – open and closed method
Open method (Open Gloving): Hands are disinfected and uncovered; the glove is applied directly onto the hand. Care must be taken not to contaminate the sterile inner surface.
Closed method (Closed Gloving): Standard practice in the operating theatre. Hands remain tucked inside the gown sleeves during donning; the glove is slid over the sleeves. This technique significantly reduces the risk of contamination.
3.4 Removal (aseptic doffing technique)
Proper removal is as important as sterile donning, since the external surface may be contaminated with bodily fluids, microorganisms or surgical residues.
Core principle: The external surface must never be touched with bare hands.
Procedure:
- Using the gloved hand, grasp the glove at the wrist and carefully roll it off from the inside out. The removed glove remains as a “package” in the gloved hand.
- With a finger of the now bare hand, slide under the cuff of the second glove and carefully peel it off outward so that the first glove is automatically enclosed within it.
- After removal, hygienic hand disinfection is mandatory.
This technique reliably prevents contact between skin and the potentially contaminated outer glove surface.
3.5 Disposal – requirements under infection control regulations
Sterile surgical gloves are considered potentially infectious after use and must be disposed of according to regulations for medical waste.
Principles of disposal:
- Discard into approved containers for contaminated medical waste
- Clear labelling of the waste container (e.g. “Infectious Waste”)
- No crushing or manual compaction
- Regular, securely sealed removal of waste
Depending on national or regional regulations, this often corresponds to categories requiring special handling. By careful doffing and proper disposal, the spread of pathogens can be prevented, safeguarding personnel, patients and the environment.
4. Standards & certifications
Sterile surgical gloves are subject to strict European requirements. These standards and legal regulations ensure that the product provides a reliable barrier during procedures, functions ergonomically and is medically safe. The following standards apply to all surgical gloves — whether powdered or powder‑free.
4.1 EN 455 (Parts 1–4) – fundamental standard for medical single‑use gloves
EN 455 is the central standard for medical gloves and defines essential performance and safety requirements. It consists of four parts:
- EN 455-1 – Integrity: Tests whether the glove forms a reliable barrier against fluids and microorganisms.
- EN 455-2 – Physical properties: Specifies requirements for tear resistance, wall thickness, elasticity and test methods.
- EN 455-3 – Biological evaluation: Governs labelling and testing for proteins, allergens and potentially extractable substances.
- EN 455-4 – Shelf life: Determines requirements for minimum shelf life and storage stability.
For sterile surgical gloves, compliance with all four parts is mandatory.
4.2 EN ISO 374 – chemical protection (Type C)
Many facilities also require basic chemical protection for surgical gloves. The EN ISO 374 standard classifies gloves according to their resistance to chemicals and microorganisms.
For surgical gloves, a baseline classification of Type C is typically relevant. This certifies that the glove resists at least one test acid for a certain period.
Important: Surgical gloves primarily serve as medical barrier products, not as highly specialised chemical‑resistant gloves. Nonetheless, this standard is important to make minimum material resistance transparent.
4.3 MDR (EU) 2017/745 – current legislation for medical devices
The Medical Device Regulation (MDR) has been the binding regulatory framework for medical devices in the EU since May 2021. Surgical gloves are classified under Risk Class IIa.
Main requirements under MDR:
- More comprehensive technical documentation
- Proof of clinical benefit and safety
- Complete traceability (UDI system)
- Manufacturer’s extended responsibility throughout the product lifecycle
- Significantly higher requirements for post‑market surveillance
Surgical gloves may only be placed on the market with a valid MDR certification.
4.4 Directive 93/42/EEC – significance & transitional provisions
The Directive 93/42/EEC (Medical Device Directive – MDD) was the regulatory basis for medical device approval in Europe for many years. Although it has been replaced by MDR, it still remains relevant in certain contexts:
- Products certified before the cut‑off date may remain on the market under defined conditions.
- Existing certificates retain their transitional validity, provided they were renewed timely and the manufacturer meets MDR‑compliant obligations for surveillance and documentation.
- Many technical tests have been transferred from MDD to MDR — hence the directive remains relevant in practice, especially for evaluating older product generations.
For sterile surgical gloves, this means: Older products with valid certificates under 93/42/EEC may continue to be used under legally regulated transitional provisions; new products, however, must fully comply with MDR.
Why standards and certifications are so important for users: For medical personnel, these standards ensure reliable barrier protection, ergonomic usability, skin compatibility, transparent performance data and legal safety. For operators such as hospitals, clinics, surgical centres and care facilities, they provide a clear foundation for procurement, quality management and documentation obligations.
5. Benefits of sterile single‑use surgical gloves
Sterile surgical gloves fulfill a safety‑critical function in the operating theatre. They protect not only medical personnel, but above all patients from contamination, pathogen transmission and undesirable interactions. Their special properties distinguish them clearly from non‑sterile examination gloves and are tailor‑made for the demands of surgical procedures.
5.1 Maximum barrier protection against pathogens
Surgical gloves are developed and tested to provide a reliable, microbiologically tight barrier. This protects against blood and other potentially infectious fluids, microorganisms and particles that may arise during surgery. The combination of material quality, controlled production and validated sterilisation ensures an exceptionally high level of protection.
5.2 Precision and tactile sensitivity
A significant advantage of surgical gloves — particularly latex gloves — is the very good tactile sensitivity. This is essential for delicate tissue preparation, precise instrument handling, suturing techniques and manipulation of sensitive structures. The anatomical pre‑shape (left/right) and optimised wall thickness enable precise work even in confined anatomical areas.
5.3 Ergonomic fit for long procedures
Surgical procedures can last several hours. Gloves are therefore designed for long‑term comfort: The anatomical shape reduces fatigue, high elasticity provides pleasant freedom of movement, the extended cuff prevents slipping under the surgical gown, and precise size gradations allow a perfectly fitting choice. A good fit prevents pressure points and improves control of hand movements.
5.4 Safety through double‑gloving (double‑glove technique)
Many surgical disciplines use a double‑glove technique to further enhance barrier protection. This adds extra safety in case of perforations, increases the likelihood of detecting punctures, and significantly reduces the risk of exposure to blood and secretions. Modern surgical gloves are designed so that wearing two gloves does not substantially impair tactile sensitivity.
5.5 High material resistance and reliable tear strength
Surgical gloves must withstand mechanical stress throughout the procedure – from contact with instruments, gripping and holding tension to moisture and fluids. High‑quality surgical gloves possess strong tear and puncture resistance without compromising mobility.
5.6 Skin‑friendliness through modern manufacturing processes
Powder‑free surgical gloves – now the standard – significantly reduce the risk of skin irritation and sensitisation reactions. Additional polymer coatings make donning easier and improve moisture management inside the glove.
5.7 Reliable quality through standards & legal requirements
Compliance with EN 455, EN ISO 374, MDR and, where applicable, still valid certificates under 93/42/EEC guarantees reproducible quality, safety over the product lifecycle, transparent performance data and clinically tested properties. The user can rely on each glove meeting defined minimum requirements and undergoing rigorous testing.
6. Typical application areas of sterile surgical gloves
Sterile surgical gloves are employed wherever complete sterility and utmost precision are essential. Their utility, however, extends beyond the classical operating theatre. They form the central protective barrier in all surgical disciplines and numerous adjacent fields where aseptic conditions or highly sensitive instrument work are required.
In hospital-based surgical operations, sterile gloves are standard equipment for every operation – regardless of specialty, duration or complexity. They protect both the surgical team and patients from contamination by blood, body fluids or microorganisms. Especially in open surgeries, minimally invasive procedures or microsurgical operations, the combination of sterile protection and tactile sensitivity is crucial for controlled, fatigue‑free work.
Also in outpatient surgery, such as in smaller surgical centres, clinics or dermatological practices, sterile surgical gloves are an integral part of hygienic workflows. They enable safe conduct of procedures such as excisions, wound closures, minimally invasive treatments or care of complex injuries. Here, rapid sterile glove availability — supported by standardised packaging and defined donning techniques — plays a key role.
In dental and oral surgery, sterile surgical gloves facilitate safe manipulation in a sensitive, hard-to-access working field. Especially during implantations, bone augmentations or extensive extractions, the combination of sterile protection and fine tactile control is indispensable to reduce infection risk and maintain surgical precision.
Beyond human medicine, sterile surgical gloves are increasingly used in veterinary medicine — especially in clinics and specialised practices performing surgical procedures on small animals or larger species. The aseptic and barrier requirements largely correspond to those in human medicine, making medical-grade surgical gloves an indispensable tool in this sector as well.
Another domain of use lies in clean‑room-like or laboratory environments, where both protection of the operator and of the material is critical. In such settings, sterile gloves are used for sensitive preparations, work on cell cultures or sterile experimental setups — wherever even minimal contamination could compromise results or pose risks.
Across all these application areas, sterile surgical gloves are always part of a comprehensive hygiene concept. They do not replace other protective measures, but complement them with a highly sensitive, anatomically optimised and reliable barrier — indispensable for sterile and controlled working conditions.